
Sf6 Polar Atau Nonpolar Brain
So, the final answer is: $\textbf{The molecule $\mathrm{SF}_{6}$ is nonpolar due to the symmetrical arrangement of polar bonds which cancel out each other's dipole moments. However, $\mathrm{SF}_{5} \mathrm{Br}$ is polar because the presence of a non-equivalent bond results in a net dipole moment for the molecule.}$

Sf6 Polar Atau Nonpolar Brain
1. The bromine atom has seven valence electrons, and each fluorine has seven valence electrons, so the Lewis electron structure is. Three fluorines are bonded to a central bromine. Each fluorine has three lone pairs, Bromine has two lone pairs. Once again, we have a compound that is an exception to the octet rule. 2.

Is SF6 (Sulfur hexafluoride) Polar or NonPolar? YouTube
Chemistry Kay B. asked • 11/03/20 Is HCN and SF6 polar or nonpolar? 1.) How to get the electronegativity difference of HCN? (I am not sure how to get the EN difference of 3 elements in a compound). 2.) Is HCN polar or nonpolar? 3.) SF 6 has an EN difference of 1.5, right? So, is it polar or nonpolar? Thank you in advance! Follow • 1 Add comment

Sf6 Polar Or Nonpolar Polar and Nonpolar Molecules Is it Polar or Nonpolar What are
Draw Lewis dot (electron) structure for SF6 and determine the following: a) electron geometry b) molecular geometry c) central atom hybridization d) polar or nonpolar This problem has been solved! You'll get a detailed solution from a subject matter expert that helps you learn core concepts.

Sf6 Polar Atau Nonpolar Brain
This answer is: More answers Wiki User ∙ 14y ago Copy SF6 is non-polar. Reason: Fluorine is more electronegative than sulfur, so the bond dipoles point toward fluorine. However, all the six S-F.

Sf6 Polar Atau Nonpolar Brain
The molecule is symmetric. The two oxygen atoms pull on the electrons by exactly the same amount. Propane is nonpolar, because it is symmetric, with H atoms bonded to every side around the central atoms and no unshared pairs of electrons. Exercise 4.12. 1. Label each of the following as polar or nonpolar.
Sf6 Polar or Nonpolar Jessica McLean
SF6 Lewis Structure has six single bonds surrounding the Sulphur atom, which has six fluorine atoms linked to it and three lone pairs on each fluorine atom while there is no lone pair on the Sulphur atom.. As a result, sulfur hexafluoride is non-polar. Factors affecting polarity. Electronegativity; The stronger the atom's ability to.
Sf6 Polar or Nonpolar RachaelkruwKramer
1. Another non polar molecule shown below is boron trifluoride, BF 3. BF 3 is a trigonal planar molecule and all three peripheral atoms are the same. Figure 4.12.1 4.12. 1 Some examples of nonpolar molecules based on molecular geometry (BF 3 and CCl 4 ). Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom.
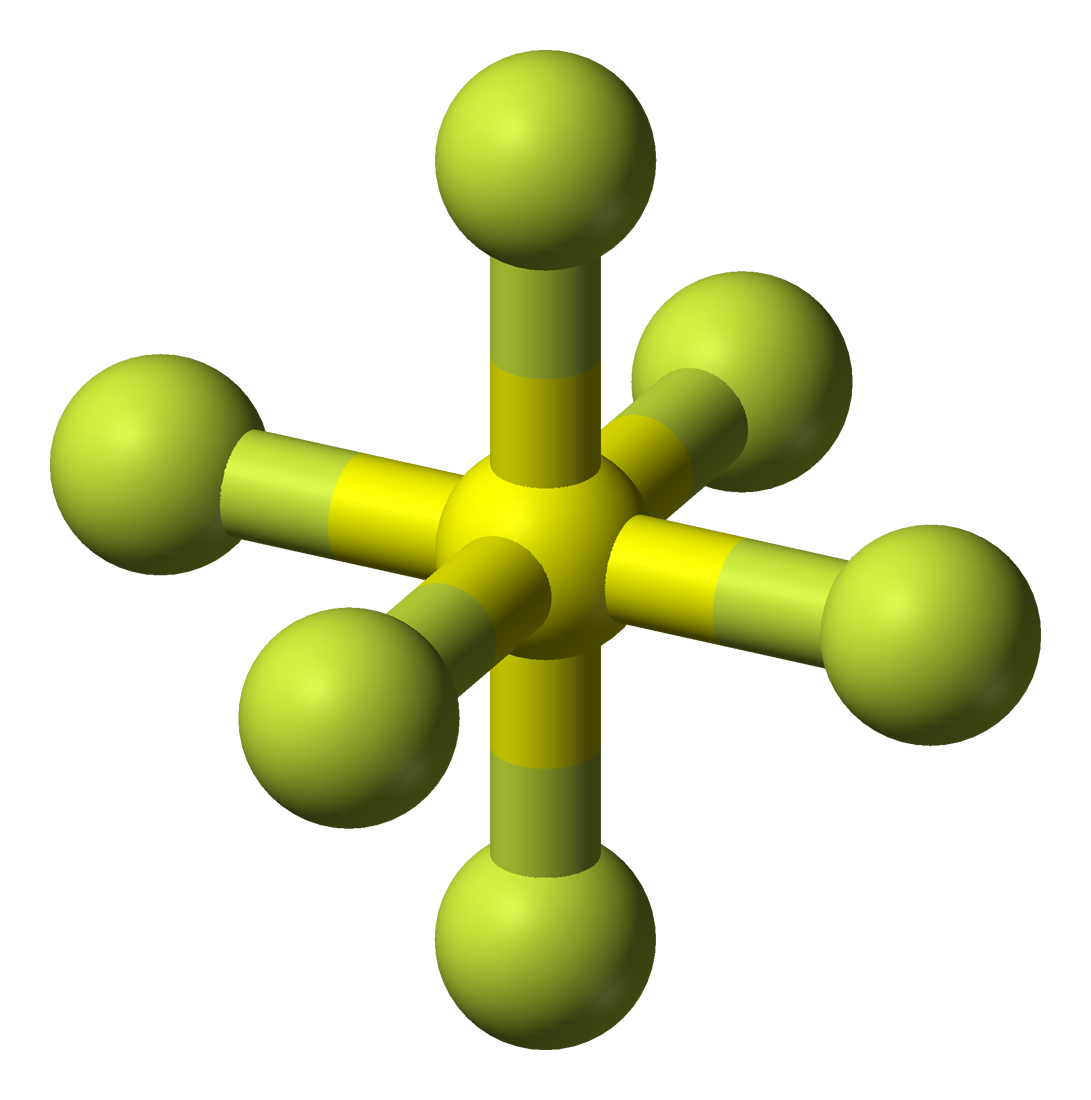
SF6 Molecular Geometry, Lewis Structure, Shape, and Polarity
Page Contents show Why SF6 is a nonpolar molecule? A covalent bond is polar if it is composed of two dissimilar atoms that have an electronegativity difference greater than 0.5 units, at least. All polar bonds have a specific dipole moment value.

Sf6 Polar Atau Nonpolar Brain
(Sulfur Hexafluoride) Geometry of Molecules 2.02K subscribers Subscribe 2 416 views 1 year ago Polarity of Molecules Hi Guys! In this video, we are going to look at the polarity of the SF6.

Sf6 Polar Atau Nonpolar Brain
Contents SF6 Valence Electrons SF6 Lewis Structure SF6 Hybridization SF6 Bond angle SF6 Molecular Geometry SF6 Shape Is SF6 polar or nonpolar? SF6 Valence Electrons To determine the Lewis Structure of any molecule, we first need to know the total number of valence electrons.

Sf6 Polar Atau Nonpolar Brain
Is S F 6 a polar or nonpolar molecule? Explain. Question: Is S F 6 a polar or nonpolar molecule? Explain. Polar Molecule: Smaller covalent molecules often only contain a single central.

Sf6 Polar Atau Nonpolar Brain
SF6 is a NONPOLAR molecule because all the six bonds (S-F bonds) are identical and SF6 has symmetrical geometry which cancels out the bond polarity. Let me explain this in detail with the help of SF6 lewis structure and its 3D geometry. Why is SF6 a Nonpolar molecule? (Explained in 3 Steps)

MakeTheBrainHappy Is SF6 Polar or Nonpolar?
Learn to determine if SF6 (Sulfur hexachloride) is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis.

Sf6 Polar or Nonpolar RachaelkruwKramer
SF6 (Sulfur hexafluoride) is a covalent (nonpolar covalent) compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Here, S is a nonmetal and F is also a nonmetal. So when they combine, it forms a covalent compound.

MakeTheBrainHappy Is SF6 Ionic or Covalent?
SF6 is a nonpolar compound in nature because as per VSEPR theory six fluorine atoms are arranged symmetrically with the sulfur atom such that dipole moment of S-F bond gets canceled out making the SF6 a nonpolar compound. SF6 has its IUPAC name Sulfur hexafluoride and it is considered as an extreme gas responsible for the greenhouse effect.